Roche Ramps Up Breast Cancer Pipeline With Regor's CDK Inhibitors
Roche RHHBY announced an asset purchase agreement with clinical-stage biotechnology company Regor Therapeutics.
Per the agreement, Roche’s Genentech will acquire Regor’s portfolio of next-generation CDK inhibitors for the treatment of breast cancer.
The purchase agreement was announced at Roche’s Pharma Day.
Year to date, shares of Roche have gained 10.3% compared with the industry’s 20.4% growth.
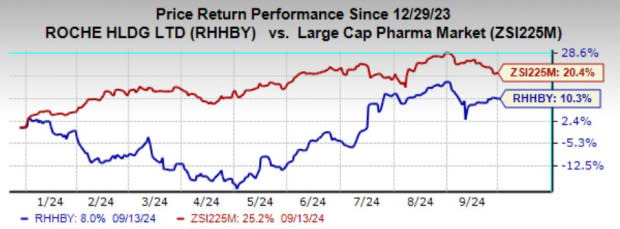
Image Source: Zacks Investment Research
Details of Roche’s Agreement With Regor
Per the terms of the deal, Regor will receive an upfront cash payment of $850 million, along with additional milestone payments.
Regor’s most advanced orally-administered CDK4/2 inhibitor in the clinic, RGT-419B, will be added to Roche’s pipeline.
RGT-419B is a potent CDK4 inhibitor with increased activity on CDK2 addressing a key mechanism of resistance to existing therapies.
Genentech will be responsible for clinical development, manufacturing and commercialization of the candidate worldwide. Regor will continue to manage the two ongoing phase I studies to their completion.
RGT-587, a phase I ready brain-penetrant selective CDK4i, will also be added to Roche’s pipeline.
Roche has a solid breast cancer franchise and the acquisition of Regor’s CDK inhibitors should strengthen its franchise further.
Two promising candidates in Roche’s breast cancer pipeline are giredestrant and inavolisib, among others. Inavolisib is currently under review in the United States with a target action date of Nov. 27, 2024.
Among CDK inhibitors, Novartis’ NVS Kisqali has witnessed a solid uptake in the breast cancer space.
Novartis recently won FDA approval for Kisqali for a broader population. The regulatory body approved Kisqali, in combination with an aromatase inhibitor, for the adjuvant treatment of people with hormone receptor-positive/human epidermal growth factor receptor 2-negative (HR+/HER2-) stage II and III early breast cancer at high risk of recurrence, including those with node-negative disease.
Other Highlights of Roche’s Pharma Day
Roche announced that 20 drugs will be launched by the company from 2020 to 2029. The company’s core focus areas are oncology and hematology, ophthalmology, neurology, immunology and cardiovascular renal and metabolic diseases.
Roche plans to cut the research and development cost of each new drug by 20%.
Roche will “fast-track” selected programs based on exceptional potential. These include an anti-TL1A inflammatory bowel disease candidate in phase III, another late-stage candidate, trontinemab, for Alzheimer’s disease and a phase II/III candidate CT-388 for obesity.
Roche targets its overall R&D spending to be roughly flat in the short term.
Roche’s shares took a hit earlier this month after it presented results from an early-stage study on its experimental obesity drug, CT-996, on concerns of possible side effects.
As per a report from Reuters, all 25 participants experienced mild or moderate side effects or adverse events, including those who only received an ineffective placebo. The side effects were mostly gastrointestinal, such as nausea, vomiting and diarrhea.
While Roche believes that these side effects can be offset by gradually ramping up the dose of the drug, the most concerning factor is the high number of adverse events.
RHHBY’s shares also took a beating after the company released data on its other experimental obesity drug CT-388, which also showed side effects, including nausea, vomiting and diarrhea.
Roche forayed into the obesity market when it acquired privately owned Carmot Therapeutics for $2.7 billion. The acquisition added a differentiated incretin portfolio with three candidates — CT-388, CT-996 and CT-868 — to its pipeline.
Roche is a pretty late entrant in the obesity market, which is currently one of the most lucrative spaces in the healthcare sector, dominated by bigwigs like Novo Nordisk NVO and Eli Lilly LLY. The stupendous success of Novo Nordisk’s obesity drug, Wegovy, puts the spotlight on the obesity space.
The FDA approved Weogovy in 2021 for chronic weight management in obese or overweight adults. Since the approval, sales of the drug have been rising consistently, driven by increased demand.
Eli Lilly’s Zepbound also witnessed a strong uptake, owing to solid demand.
Roche’s Zacks Rank
Roche currently has a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Novartis AG (NVS) : Free Stock Analysis Report
Roche Holding AG (RHHBY) : Free Stock Analysis Report
Novo Nordisk A/S (NVO) : Free Stock Analysis Report
Eli Lilly and Company (LLY) : Free Stock Analysis Report