Regeneron, SNY's Dupixent Get CHMP Recommendation for Label Expansion
Regeneron Pharmaceuticals, Inc. REGN announced that the blockbuster asthma drug Dupixent (dupilumab) has been recommended by the European Medicines Agency’s Committee for Medicinal Products for Human Use ("CHMP") for approval for an additional indication.
The CHMP recommended the label expansion of Dupixent in the European Union (EU) for eosinophilic esophagitis (EoE) in children down to 1 year of age. The recommendation is for children aged 1 to 11 years who weigh at least 15 kg and are inadequately controlled by, intolerant to, or are not candidates for conventional medicinal therapy.
Regeneron’s shares have rallied 30.9% year to date compared to the industry’s breakeven performance.
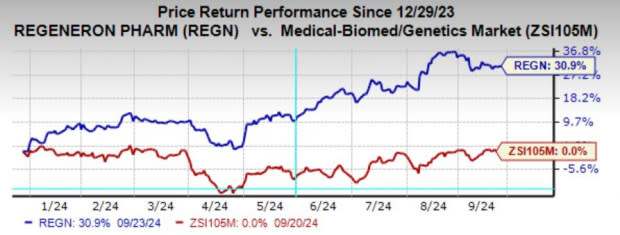
Image Source: Zacks Investment Research
The company has a collaboration agreement with Sanofi SNY for Dupixent.
Sanofi records global net product sales of Dupixent, while Regeneron records its share of profits/losses in connection with the global sales of both drugs.
CHMP's Positive Opinion for SNY/REGN’s Dupixent
The positive CHMP opinion is supported by a two-part (Part A and B) phase III study in children aged 1 to 11 years.
In Part A, a significantly greater proportion of children receiving weight-based doses of Dupixent achieved histological disease remission at week 16, compared to placebo, with results sustained for up to one year in Part B.
The safety results were generally consistent with the known safety profile of Dupixent in adolescents and adults with EoE.
A final decision from the EC is expected shortly. Dupixent is already approved in the EU for certain adults and adolescents aged 12 years and older with EoE.
EoE is a chronic, progressive disease associated with type 2 inflammation which mostly damages the esophagus and impairs its function.
Profits From Dupixent Boost REGN’s Top Line
Dupixent is already approved in several countries for certain patients with atopic dermatitis, asthma, chronic rhinosinusitis with nasal polyps (CRSwNP), EoE, prurigo nodularis, chronic spontaneous urticaria (CSU) and chronic obstructive pulmonary disease in different age populations.
Regeneron and Sanofi are evaluating dupilumab in late-stage studies for chronic pruritus of unknown origin and bullous pemphigoid.
The label expansion of Dupixent should increase REGN’s profit and boost its top line.
The FDA recently approved Dupixent for the indication of CRSwNP for a broader population. The regulatory body approved the drug as an add-on maintenance treatment for adolescent patients aged 12 to 17 years with inadequately controlled CRSwNP.
Dupixent maintains its stellar performance and profits from this drug have helped REGN in offsetting the declining sales of its lead drug, Eylea.
Eylea sales continue to be under pressure due to competition from Roche’s RHHBY Vabysmo. Roche has designed Vabysmo to block pathways involving Ang-2 and VEGF-A. The drug raked in sales of CHF 1.8 billion in the first half of 2024 on strong demand in all regions.
Roche has already obtained FDA approval for Vabysmo prefilled syringe for people living with wet age-related macular degeneration, DME and retinal vein occlusion.
Zacks Rank
Regeneron currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Regeneron Pharmaceuticals, Inc. (REGN) : Free Stock Analysis Report
Sanofi (SNY) : Free Stock Analysis Report
Roche Holding AG (RHHBY) : Free Stock Analysis Report