Nuvalent Stock Moves Up 20% in a Week: What's Driving the Rally?
Shares of Nuvalent NUVL have soared nearly 20% in the past week owing to encouraging updates on its two investigational therapies. Both therapies target previously treated non-small cell lung cancer (NSCLC) patients but focus on different genetic mutations.
These updates were announced earlier this weekend at the European Society for Medical Oncology (ESMO) Congress 2024 meeting held in Spain.
NUVL Boasts Encouraging Pipeline Potential in NSCLC Space
Management presented updated data from the early-stage portions of two separate phase I/II studies — ARROS-1 and ALKOVE-1. While the ARROS-1 study evaluates zidesamtinib inpatients with advanced ROS1-positive NSCLC, the ALKOVE-1 study evaluates NVL-655 in patients with advanced ALK-positive NSCLC.
Data from the ARROS-1 study showed that 44% of patients who received zidesamtinib responded to treatment. In the ALOVE-1 study, 38% of patients responded to treatment with NVL-655.
Both studies involved patients whose cancers failed to respond to heavily pre-treated patients who had already been treated with Pfizer’s PFE Lorbrena or Bristol Myers’ BMY Augtyro. While the PFE drug is approved to treat ALK-positive NSCLC patients, the BMY drug is approved for ROS1-positive NSCLC.
These updates impressed investors, which sent Nuvalent’s stock price soaring. Several Wall Street analysts supported the company’s claims that both drugs could be the best-in-class therapies in their respective categories. Per management, zidesamtinib and NVL-655 demonstrate the potential for treating third-line NSCLC patients — an area where no approved therapies have demonstrated clinical benefit. The drugs also provide a differentiated option to NSCLC patients in the second-line setting.
Year to date, Nuvalent’s shares have surged 42.4% compared with the industry’s 1.0% growth. During this timeframe, the stock has also outperformed the sector and the S&P 500. The company’s shares are also trading above the 50-day and 200-day moving averages.
NUVL Stock Outperforms Industry, Sector & S&P 500
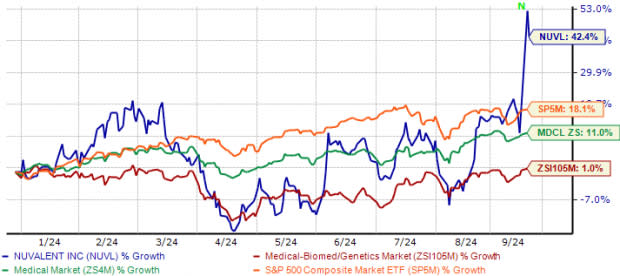
Image Source: Zacks Investment Research
Nuvalent’s Future Plans for Both Drugs
Alongside results, management also provided updates on its plans and development strategies for both candidates. Currently, NUVL is evaluating both drugs in the phase II portions of the ARROS-1 and ALKOVE-1 studies. These mid-stage portions have been designed with registrational intent. If successful, management could seek accelerated approval from the FDA for the drugs. Data from these portions is expected next year.
These results are likely to boost investors’ confidence in the company’s OnTarget 2026 operating plan announced earlier this year, which lays down management’s path toward a potential first approval in 2026.
Management also announced plans for a late-stage study, which will evaluate NVL-655 against Roche’s RHHBY Alecensa in ALK-positive NSCLC patients who have not received any TKI inhibitors.
Roche’s Alecensa is approved to treat adult patients with ALK-positive metastatic NSCLC. In April, the FDA granted label expansion to this RHHBY drug for adjuvant treatment following tumor resection in ALK-positive NSCLC patients.
Nuvalent, Inc. Price
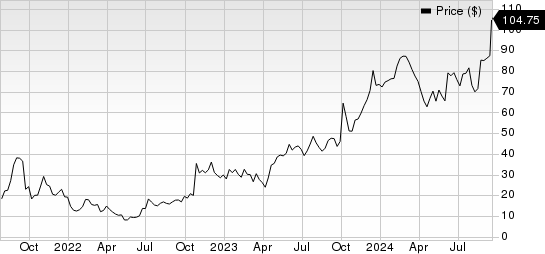
Nuvalent, Inc. price | Nuvalent, Inc. Quote
NUVL’s Zacks Rank
Nuvalent currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank stocks here.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Roche Holding AG (RHHBY) : Free Stock Analysis Report
Bristol Myers Squibb Company (BMY) : Free Stock Analysis Report
Pfizer Inc. (PFE) : Free Stock Analysis Report
Nuvalent, Inc. (NUVL) : Free Stock Analysis Report