Incyte Announces Promising New Data on Oncology Candidate at ESMO
Incyte INCY announced new data from a phase I study on pipeline candidate INCB123667 in patients with advanced solid tumors.
INCB123667 is a highly selective, potential first-in-class CDK2 inhibitor.
The new data was presented during a mini-oral presentation at the European Society of Medical Oncology.
The updated data highlight the potential of the candidate as a differentiated treatment option for cancers with increased cyclin E1 activity, amplification and/or overexpression in cells predictive of CDK2 dependency.
Shares of Incyte have risen 3.1% year to date compared with the industry’s growth of 0.5%.
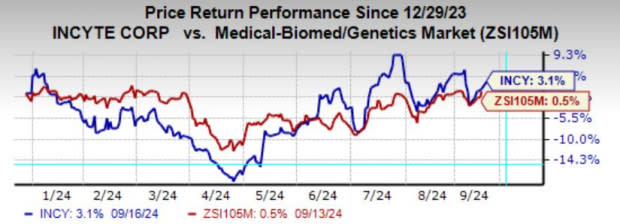
Image Source: Zacks Investment Research
Incyte’s Promising INCB123667 Data
This open-label, dose-escalation and dose-expansion phase I study is evaluating the safety, tolerability, pharmacokinetics, pharmacodynamics and preliminary efficacy of INCB123667 when administered as monotherapy at the recommended dose for expansion (RDE[s]) in participants with selected advanced or metastatic solid tumors.
While part 1A (dose escalation) determined the recommended dose of INCB123667 for expansion and the maximum tolerated dose (MTD), part 1B (cohort dose expansion phase) will further explore antitumor activity of INCB123667 as a monotherapy in six tumor-specific cohorts at the RDEs defined in part 1A.
The study enrolled patients with advanced or metastatic solid tumors (n=205), including ovarian cancer, endometrial cancer, gastrointestinal cancer, HR+/HER2- breast cancer and triple negative breast cancer, among others. These patients received varying doses of INCB123667, from 50mg to 150mg using once-daily (QD) and twice-daily (BID) dosing schedules.
Data from the phase Ib dose expansion portion of the trial (data cut-off August 26, 2024) showed single-agent antitumor activity, and decreases in circulating tumor DNA (ctDNA) across a range of doses and regimens. The antitumor activity was most evident in patients with ovarian cancer and endometrial cancer whose tumors overexpress cyclin E1. The trial is ongoing and the data will continue to mature.
Nine participants of the 37 evaluable participants with platinum-resistant ovarian cancer treated at three selected dose levels (50mg BID, 100mg QD and 125mg QD) in this portion of the trial experienced an overall response (OR). Two of them achieved complete responses (CR) and seven obtained partial responses (PRs).
The highest OR rate of 31.3% was found in the 50mg BID cohort. Additionally, a disease control rate of 75.7% was achieved in patients with ovarian cancer. Four PRs were reported among patients with endometrial cancer.
The part Ib data built on results from the dose escalation portion (part 1a) of the study evaluating the safety and tolerability of INCB123667, which demonstrated a manageable safety profile.
INCY plans to initiate a pivotal study in ovarian cancer in 2025 and evaluate INCB123667 in combination with other treatments.
INCY Announces Data on Zynyz
Incyte also announced results from the late-stage POD1UM-303/InterAACT2 study of retifanlimab.
The study is evaluating retifanlimab, a humanized monoclonal antibody targeting programmed death receptor-1 (PD-1), in combination with platinum-based chemotherapy (carboplatin–paclitaxel) for the treatment of adults with inoperable locally recurrent or metastatic squamous cell anal carcinoma (SCAC).
The study met primary endpoint of progression-free survival and demonstrated improvement across secondary endpoints in patients with SCAC taking retifanlimab in combination with platinum-based chemotherapy (carboplatin-paclitaxel).
The data support the planned filing of a supplemental biologics license application for retifanlimab in SCAC by year-end 2024.
Retifanlimab is already approved in the United States under the brand name Zynyz in the United States for the treatment of adult patients with metastatic or recurrent locally advanced merkel cell carcinoma.
INCY’s Efforts to Develop Pipeline
Last month, Incyte and partner Syndax Pharmaceuticals SNDX obtained FDA approval for axatilimab-csfr, an anti-CSF-1R antibody, for the treatment of chronic graft-versus-host disease (GVHD) after the failure of at least two prior lines of systemic therapy in adult and pediatric patients weighing at least 40 kg.
The candidate was approved under the brand name Niktimvo.
Syndax and Incyte collaborated on the worldwide co-development and co-commercialization license agreement for axatilimab in chronic GVHD and any future indication.
The drug will be co-commercialized by Incyte and Syndax Pharmaceuticals in the United States, while Incyte has exclusive commercialization rights for Niktimvo outside of the country.
Approval of new drugs and a potential label expansion of additional drugs are important for Incyte as the company is heavily dependent on its lead drug, Jakafi (ruxolitinib), for its top-line growth.
INCY’s Rank & Stocks to Consider
Incyte currently carries a Zacks Rank #3 (Hold).
A couple of better-ranked stocks from the biotech sector are Krystal Biotech, Inc. KRYS and Fulcrum Therapeutics, Inc. FULC, both sporting a Zacks Rank #1 (Strong Buy) at present. You can see the complete list of today’s Zacks #1 Rank stocks here.
In the past 60 days, estimates for Krystal Biotech’s 2024 earnings per share have increased from $1.98 to $2.38. Earnings per share estimates for 2025 have improved from $4.33 to $7.31 during the said time frame. Year to date, shares of KRYS have risen 59.9%.
In the past 60 days, estimates for Fulcrum Therapeutics’ 2024 loss per share have narrowed from $1.33 to 32 cents. Loss per share estimate for 2025 has narrowed from $1.71 to $1.22 during the same time frame.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Incyte Corporation (INCY) : Free Stock Analysis Report
Syndax Pharmaceuticals, Inc. (SNDX) : Free Stock Analysis Report
Krystal Biotech, Inc. (KRYS) : Free Stock Analysis Report
Fulcrum Therapeutics, Inc. (FULC) : Free Stock Analysis Report