GSK's HSV Vaccine Study Fails to Meet Primary Efficacy Goal
GSK plc GSK announced that its therapeutic herpes simplex virus (HSV) vaccine candidate, GSK3943104, did not meet the primary endpoint in the combined phase I/II proof-of-concept study.
The phase I/II TH HSV REC-003 study evaluated the clinical efficacy of the early-stage HSV vaccine candidate GSK3943104.
The company completed the primary objective data analysis from the phase II part of the phase I/II TH HSV REC-003 study, which demonstrated that GSK3943104 failed to meet the study's primary efficacy endpoint.
Following this, the company decided not to progress with phase III development of GSK3943104.
Shares of GSK have rallied 18.1% so far this year against the industry's decline of 1%.
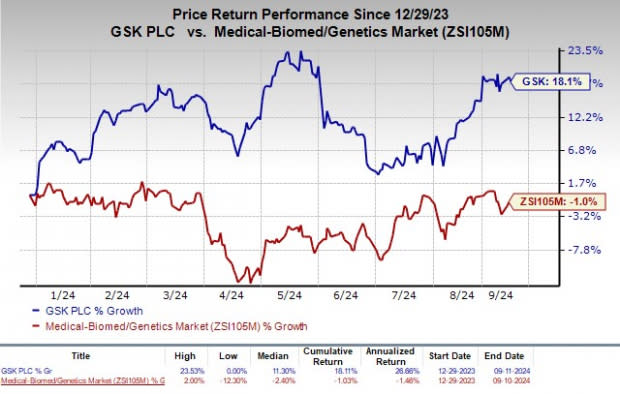
Image Source: Zacks Investment Research
Positive Data From GSK’s mRNA-based Flu Vaccine Program
In a separate press release, GSK announced positive headline data from a phase II study that evaluated certain mRNA-based seasonal influenza vaccine candidates.
The study investigated various mRNA formulations of the seasonal influenza vaccine candidates, which can improve immune responses against influenza A and B strains in older and younger adults versus the current standard of care.
The data showed that mRNA formulations in one of the vaccine candidates demonstrated positive A and B strain immune responses relative to the standard of care in both older and younger adults.
Per the company, the above data shows that the mRNA platform elicits strong overall antibody titers with an acceptable safety profile.
GSK’s seasonal influenza vaccine candidate is currently in phase II development. Building on the success of the above data, the company will now proceed into phase III development of the vaccine candidate.
GSK’s New Licensing Agreement With CureVac
GSK restructured its existing collaboration with German vaccine maker, CureVac CVAC into a new licensing agreement in July 2024.
Per the terms of the new agreement, GSK will assume full control of developing and manufacturing mRNA candidate vaccines for influenza and COVID-19, along with commercialization rights. CVAC retains exclusive rights to the additional undisclosed and preclinically validated infectious disease targets from the prior collaboration.
In addition, CureVaC has the liberty to independently develop and partner mRNA vaccines in any other infectious disease or other indication.
Zacks Rank & Stocks to Consider
GSK currently carries a Zacks Rank #3 (Hold).
Some better-ranked stocks in the biotech sector are Krystal Biotech, Inc. KRYS and Fulcrum Therapeutics, Inc. FULC, each sporting a Zacks Rank #1 (Strong Buy) at present. You can see the complete list of today’s Zacks #1 Rank stocks here.
In the past 60 days, estimates for Krystal Biotech’s 2024 earnings per share have increased from $1.98 to $2.38. Earnings per share estimates for 2025 have improved from $4.33 to $7.31. Year to date, shares of KRYS have risen 60.3%.
KRYS’ earnings beat estimates in three of the trailing four quarters while missing on the remaining occasion, the average surprise being 45.95%.
In the past 60 days, estimates for Fulcrum Therapeutics’ 2024 loss per share have narrowed from $1.24 to 48 cents. Loss per share estimates for 2025 have narrowed from $1.71 to $1.53. Year to date, shares of FULC have jumped 31.1%.
FULC’s earnings beat estimates in each of the trailing four quarters, the average surprise being 393.18%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
GSK PLC Sponsored ADR (GSK) : Free Stock Analysis Report
Krystal Biotech, Inc. (KRYS) : Free Stock Analysis Report
Fulcrum Therapeutics, Inc. (FULC) : Free Stock Analysis Report
CureVac N.V. (CVAC) : Free Stock Analysis Report