Bayer Presents Encouraging Data From Late-Stage Cardiovascular Study
Bayer BAYRY announced encouraging detailed results from the late-stage FINEARTS-HF study on finerenone.
Finerenone is marketed as Kerendia or Firialta in some countries. The drug is approved for the treatment of adult patients with chronic kidney disease (CKD) associated with type 2 diabetes (T2D) in more than 90 countries worldwide, including China, Europe, Japan and the United States.
It is a non-steroidal, selective mineralocorticoid receptor (MR) antagonist that has been shown to block the harmful effects of MR overactivation.
Year to date, shares of Bayer have lost 16.4% against the industry’s growth of 28.6%.
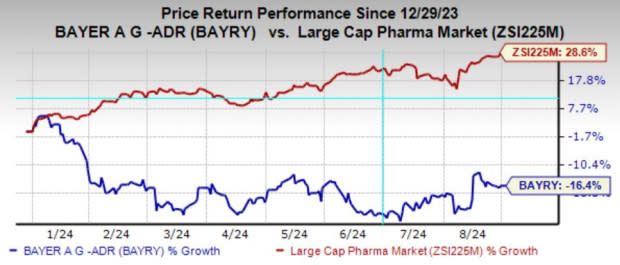
Image Source: Zacks Investment Research
BAYRY’s Cardiovascular Study on Kerendia
FINEARTS-HF is a randomized, double-blind, placebo-controlled, multicenter, event-driven phase III study investigating the efficacy and safety of finerenone for the prevention of cardiovascular death and heart failure (HF) events in patients with a diagnosis of symptomatic heart failure with a left ventricular ejection fraction (LVEF) of ≥40%.
According to detailed results from the study, finerenone had a statistically significant improvement in cardiovascular outcomes in HF patients with a LVEF of greater than or equal to 40% as compared to placebo.
Finerenone significantly reduced the risk of the composite primary endpoint of cardiovascular death and total (first and recurrent) HF events, defined as hospitalizations for HF or urgent HF visits, by 16% over a median duration of 32 months.
Per Bayer, finerenone is the first MR antagonist to demonstrate definitive cardiovascular benefits in a phase III study in patients with this common form of heart failure.
Results also showed that the benefits presented in the primary endpoint were consistent across all prespecified subgroups, regardless of background therapy, comorbidities, or hospitalization status, including those based on disease state (ejection fraction) or baseline use of SGLT2-inhibitors.
Finerenone also significantly reduced the secondary endpoints of total HF events and improved patient-reported health status as measured by the change from baseline in Total Symptom Score of Kansas City Cardiomyopathy Questionnaire.
Bayer plans to submit applications to health authorities for marketing authorization for finerenone for an indication in heart failure with a LVEF of ≥40%.
Finerenone was well-tolerated in the FINEARTS-HF study and the overall incidence of treatment-emergent serious adverse events was comparable between finerenone and placebo groups. However, hyperkalemia-related adverse events occurred more frequently with finerenone than placebo (9.7 % and 4.2%, respectively).
How Will Kerendia’s Label Expansion Help Bayer?
There is a significant need for treatments for heart failure patients with LVEF ≥ 40%. These patients have a substantial risk for serious cardiovascular events. While there are many treatments available for heart failure with reduced ejection fraction, there are limited treatment options for heart failure with LVEF ≥ 40%.
Hence, the successful development and commercialization of finerenone for this indication will be beneficial for Bayer.
The study program with finerenone, FINEOVATE, currently comprises 10 phase III studies with dedicated programs in HF and CKD.
Bayer’s Efforts to Strengthen Pharma Business
Bayer is looking to strengthen its pharmaceutical portfolio amid generic competition for Xarelto.
Sales in the Pharmaceuticals Division increased 4.5% to €4.6 billion in the second quarter of 2024, driven by the strong performance of the cancer drug Nubeqa and CKD drug Kerendia. Eylea also continues to perform well.
Bayer is working on the label expansion of its approved drugs and simultaneously developing new drugs.
The company is working to get a higher dose of Eylea approved. Bayer is also working on the label expansion of Nubeqa and Kerendia in various indications. This should further fuel sales of these drugs.
Pipeline setbacks and regulatory setbacks have weighed on the stock in recent times. In light of these challenges, Bayer is implementing a new operating model to reduce hierarchies, eliminate bureaucracy, streamline structures and accelerate decision-making processes. The company is also undertaking significant job cuts.
BAYRY’s Zacks Rank & Stocks to Consider
BAYRY currently carries a Zacks Rank #3 (Hold).
Some better-ranked stocks from the pharma/biotech industry are Eli Lilly LLY and Exelixis EXEL, both carrying a Zacks Rank #2 (Buy) at present. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Estimates for Eli Lilly’s 2024 earnings have risen from $13.71 to $15.77 per share over the past 30 days. For 2025, the bottom-line estimate has risen from $19.42 to $22.79 over the same timeframe. Year to date, Lilly’s shares have risen 64.7%.
In the past 60 days, estimates for EXEL’s 2024 earnings per share have moved north to $1.79 from $1.39. Year to date, shares of EXEL have risen 8.5%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Eli Lilly and Company (LLY) : Free Stock Analysis Report
Bayer Aktiengesellschaft (BAYRY) : Free Stock Analysis Report
Exelixis, Inc. (EXEL) : Free Stock Analysis Report