Apellis Stock Down as Eye Drug Faces Third CHMP Rejection in the EU
Apellis Pharmaceuticals APLS announced that the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) has confirmed its June 2024 negative opinion on the regulatory filing for intravitreal pegcetacoplan to treat geographic atrophy (GA) secondary to age-related macular degeneration (AMD). Shares of the company lost 11.5% on Friday in response.
Management expressed deep disappointment in the outcome, stating that millions of GA patients in the EU remain without treatment for this irreversible form of blindness. Apellis further reported that the CHMP opinion was issued despite broad support for pegcetacoplan from the European retina community and several dissenting votes from CHMP members who had advocated for a path to approval.
Prior Regulatory Updates for APLS’ Pegcetacoplan
We remind investors that the CHMP had first voted against the marketing authorization application (MAA) for Syfovre to treat GA in January 2024, following which Apellis demanded a re-examination of the same with the EMA. However, in March 2024, the Court of Justice of the European Union (CJEU) issued a judgment that ruled on the organization of EMA’s expert groups, posing implications for the EMA's policy for handling competing interests of experts.
Based on the CJEU’s judgment, the EMA had reset the review of the Syfovre MAA for the GA indication to the last phase of the initial assessment (day 180) in April 2024. Apellis had clarified that the decision to reset the Syfovre MAA review was strictly procedural in response to the CJEU judgment and did not imply any shortcoming in the submission package for the drug, which was intended to support the approval in the EU.
Following the CJEU’s judgment, in June 2024, the CHMP issued a negative opinion for the second time on the MAA for intravitreal pegcetacoplan to treat GA in the EU.
Year to date, shares of APLS have plunged 45.7% against the industry’s 0.5% growth.
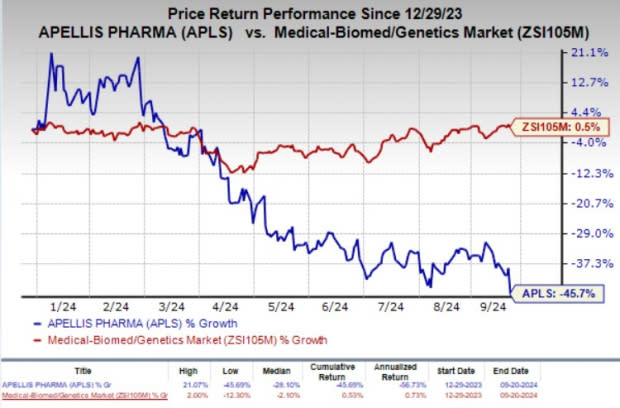
Image Source: Zacks Investment Research
APLS’ Portfolio of Marketed Drugs
Please note that pegcetacoplan injection is currently approved in the United States under the brand name Syfovre for treating GA secondary to AMD.
Syfovre was approved by the FDA in February 2023 as the first and only treatment for GA secondary to AMD in the United States. It has the potential to be a best-in-class treatment for patients with GA, a disease that is the leading cause of blindness, affecting more than one million people in the United States and five million people worldwide.
The drug has witnessed robust initial uptake since its commercial launch in March 2023. Syfovre recorded sales of $292.1 million in the first half of 2024, which increased 241% year over year, owing to continued strong demand, thereby driving revenues for the company. As of June 30, 2024, the total number of drug doses delivered since launch was 330,000.
Marketing authorization seeking applications for intravitreal pegcetacoplan for the treatment of GA is currently under review in several other countries apart from the EU, with decisions expected in the current year. Potential approval of the drug in additional geographies will further boost the top line.
Apellis also currently markets pegcetacoplan under the brand name Empaveli/Aspaveli in the United States and EU as a monotherapy treatment for adult patients suffering from paroxysmal nocturnal hemoglobinuria. The drug is also approved in several other geographies for the same indication.
Apellis Pharmaceuticals, Inc. Price and Consensus
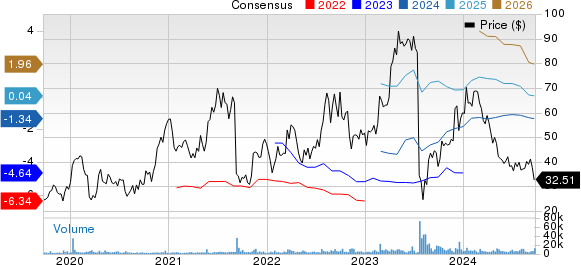
Apellis Pharmaceuticals, Inc. price-consensus-chart | Apellis Pharmaceuticals, Inc. Quote
Zacks Rank & Stocks to Consider
Apellis currently carries a Zacks Rank #3 (Hold).
Some better-ranked stocks in the biotech sector are Illumina, Inc. ILMN, Krystal Biotech, Inc. KRYS and Fulcrum Therapeutics, Inc. FULC, each sporting a Zacks Rank #1 (Strong Buy) at present. You can see the complete list of today’s Zacks #1 Rank stocks here.
In the past 60 days, estimates for Illumina’s 2024 EPS have moved up from $1.84 to $3.63. The consensus estimate for 2025 earnings has improved from $3.22 to $4.43. Year to date, shares of ILMN have lost 5.8%.
ILMN’s earnings beat estimates in each of the trailing four quarters, the average surprise being 463.46%.
In the past 60 days, estimates for Krystal Biotech’s 2024 EPS have increased from $2.09 to $2.38. The consensus estimate for 2025 earnings has improved from $4.33 to $7.31. Year to date, shares of KRYS have jumped 48.5%.
KRYS’ earnings beat estimates in three of the trailing four quarters while missing on the remaining occasion, the average surprise being 45.95%.
In the past 60 days, estimates for Fulcrum Therapeutics’ 2024 loss per share have narrowed from $1.33 to 28 cents. The consensus estimate for 2025 loss per share has narrowed from $1.71 to $1.14. Year to date, shares of FULC have plunged 50.2%.
FULC’s earnings beat estimates in each of the trailing four quarters, the average surprise being 393.18%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Illumina, Inc. (ILMN) : Free Stock Analysis Report
Krystal Biotech, Inc. (KRYS) : Free Stock Analysis Report
Apellis Pharmaceuticals, Inc. (APLS) : Free Stock Analysis Report
Fulcrum Therapeutics, Inc. (FULC) : Free Stock Analysis Report