Accelerate Diagnostics Stock Down Despite FDA Clearance for Arc System
Accelerate Diagnostics, Inc. AXDX has secured FDA 510(k) clearance for its Accelerate Arc system and BC kit, designed for use with Bruker's MALDI Biotyper CA system (MBT-CA System) and MBT-CA Sepsityper software extension.
Accelerate Diagnostics' automated platform simplifies the preparation of positive blood culture samples for direct microbial identification using Bruker's MBT-CA system, removing the overnight culture requirement. The Arc system reduces wait times for critical diagnostic results, which is essential in sepsis treatment.
Accelerate Diagnostics completed the WAVE pre-clinical trial for its innovative WAVE system, which provides rapid antimicrobial susceptibility testing (AST) directly from positive blood culture bottles and isolated colonies.With sepsis affecting an estimated 49 million people globally each year and leading to around 11 million deaths, the WAVE system aims to enhance patient outcomes by enabling timely targeted antimicrobial therapy. This rapid testing not only improves patient care but also has the potential to reduce healthcare costs and combat antimicrobial resistance, addressing a critical challenge in the healthcare system.
Likely Trend of AXDX Stock Following the News
Following the news, shares of AXDX declined 2.8% to $1.71 at yesterday’s closing.
Shares of Accelerate Diagnostics have plunged 56.4% year to date against the industry’s 8.9% growth. The S&P 500 has risen 20.5% in the same time frame.
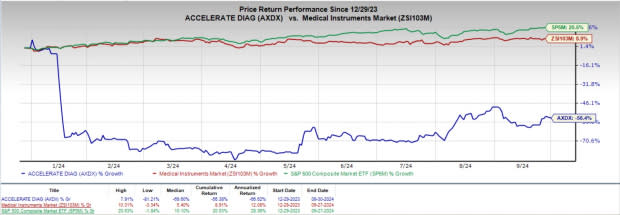
Image Source: Zacks Investment Research
Despite the recent price decline, the FDA clearance of Accelerate Diagnostics' Arc system marks a positive shift in sentiment. The company, known for addressing antibiotic resistance and sepsis, has strengthened its portfolio with the Accelerate Arc system. Along with the already FDA-approved Pheno system and PhenoTest BC kit, these technologies aim to reduce the time clinicians need to identify optimal antibiotic therapies for life-threatening infections.This recent clearance reinforces the company's commitment to enhancing patient care and boosting clinical efficiency.
Significance of AXDX’s Arc System Gaining FDA Clearance
The FDA clearance of the Accelerate Arc system represents a key advancement in rapid diagnostics, offering faster identification of bloodborne pathogens.
The Accelerate Arc system utilizes the extensive Bruker MBT-CA reference library to deliver quicker microbial identification and potentially integrate with the future Accelerate WAVE system for antibiotic susceptibility testing. The Arc system enables clinicians to administer appropriate treatments sooner, enhancing sepsis outcomes, lowering antimicrobial resistance and reducing hospital costs. This system also addresses regulatory pressures on labs to adopt FDA-cleared devices, providing an efficient, automated alternative to traditional methods.
Accelerate Arc system is designed to replace overnight subculture and laborious LDT sample preparation in clinical labs. This clearance allows labs to meet growing regulatory requirements by using an automated, FDA-approved system.The company’s broader innovation, including the Accelerate WAVE system, aims to enhance diagnostic speed and improve patient outcomes.
Market Prospects Favoring AXDX
Per a report in Precedence Research, the global blood culture test market size is estimated to be worth $6.7 billion in 2024. It is anticipated to reach $20 billion by 2034 at a CAGR of 11.5%.
The robust growth is likely to be driven by the global spread of antimicrobial resistance and focus on early disease detection.
Recent Development at Accelerate Diagnostics
In April, Accelerate Diagnostics submitted its 510(k) application to the FDA for its Accelerate Arc system and BC kit, an automated platform for rapid blood culture sample preparation. Designed for clinical labs, the system works with Bruker’s MBT-CA system, delivering microbial identification results in about 1.5 hours, significantly reducing wait times.
Zacks Rank & Other Key Picks
Currently, Accelerate Diagnostics carries a Zacks Rank #2 (Buy).
Some other top-ranked stocks in the broader medical space are Tenet HealthCare THC, ATI Physical Therapy (ATIP) and Aveanna HealthcareAVAH. While Tenet HealthCare carries a Zacks Rank #1 (Strong Buy), ATI Physical Therapy and Aveanna Healthcare carry a Zacks Rank #2 each at present. You can see the complete list of today’s Zacks #1 Rank stocks here.
Tenet HealthCare has an estimated long-term growth rate of 18.3%. THC's earnings surpassed estimates in each of the trailing four quarters, with the average being 58.5%.
Tenet HealthCare has gained 119.9% compared with the industry's 48.6% growth year to date.
ATI Physical Therapy's earnings surpassed estimates in each of the trailing four quarters, with the average surprise being 7.25%.
ATIP's shares have gained 5.5% year to date compared with the industry’s 18.6% growth.
Aveanna Healthcare's earnings surpassed estimates in each of the trailing four quarters, with the average surprise being 47.5%.
AVAH's shares have surged 104.5% year to date compared with the industry’s15.7% growth.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Tenet Healthcare Corporation (THC) : Free Stock Analysis Report
Accelerate Diagnostics, Inc. (AXDX) : Free Stock Analysis Report
Aveanna Healthcare Holdings Inc. (AVAH) : Free Stock Analysis Report
ATI Physical Therapy, Inc. (ATIP) : Free Stock Analysis Report